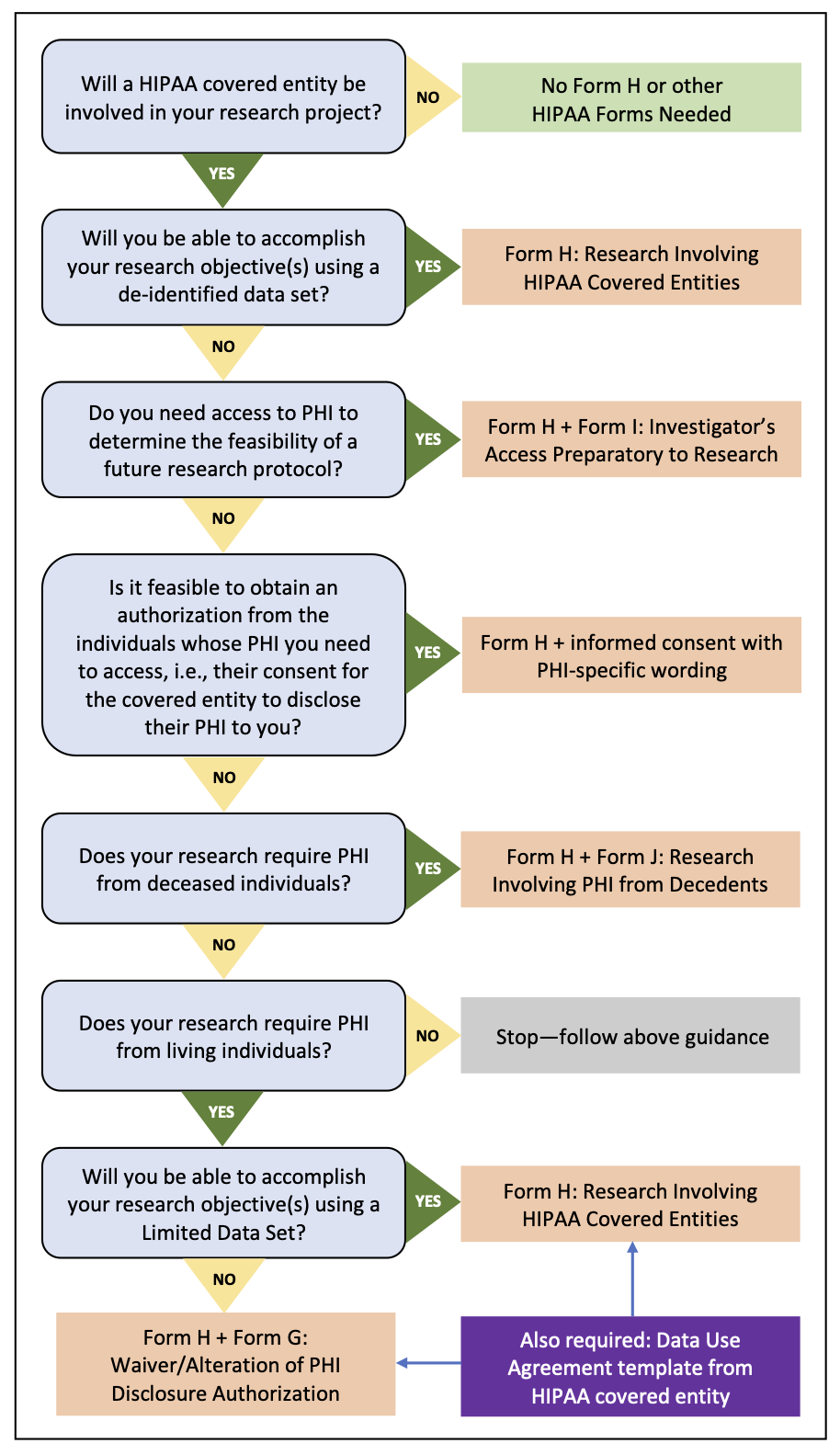
IRB Application and Review Process for Research Involving PHI
The IRB application forms are designed to help identify whether your research will involve a HIPAA covered entity, and if so, whether you will need them to disclose Protected Health Information (PHI) to you. The Protocol Application Form includes a screening question regarding involvement of HIPAA covered entities. If a covered entity will be disclosing health information to you for research purposes, you must upload Form H: Research Involving HIPAA Covered Entities to your protocol application on DASH Research IRB. This form will help you and the IRB determine whether the health information you need qualifies as PHI. If so, Form H will also help identify any additional forms or information that may be needed to facilitate IRB review and execution of a Data Use Agreement, if required. The graphic below provides a visual representation of the decision-making process regarding IRB application components.
Review Process
Most research protocols that involve use of PHI require full board (convened) review after an intake review and initial revisions are completed (if needed). Please contact the IRB Coordinator at [email protected] to identify the date of the next full board meeting.
Convened IRB review will result in one of the following outcomes:
- Approved as submitted
- Approved with contingencies – The committee has requested minor revisions or clarifications regarding the protocol. Research may not begin until contingencies are addressed. Minor revisions/contingencies can be reviewed and approved by the IRB Chair or his/her designee.
- Action deferred – The application lacks sufficient information for the committee to determine whether the criteria for approval have been met. The investigator(s) must provide additional information before the protocol can be considered further. Major revisions may require follow-up convened review by the IRB.
- Disapproval – A protocol is only disapproved if a majority of the committee believes it is not possible to modify the study design to provide adequate protection for research participants.
- Tabled due to insufficient time to review the application or lack of a quorum. The protocol will be put on the agenda for the next convened review.
If you have questions as you are planning research that will involve PHI and developing your IRB application, IRB staff are happy to assist and can be reached at [email protected].